[Latest] Global Virtual Clinical Trials Market Size/Share Worth USD 15.8 Billion by 2034 at a 5.8% CAGR: Custom Market Insights (Analysis, Outlook, Leaders, Report, Trends, Forecast, Segmentation, Growth Rate, Value, SWOT Analysis)
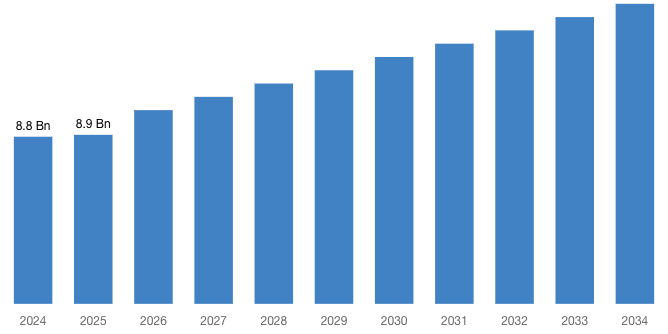
[220+ Pages Latest Report] According to a market research study published by Custom Market Insights, the demand analysis of Global Virtual Clinical Trials Market size & share revenue was valued at approximately USD 8.8 Billion in 2024 and is expected to reach USD 8.9 Billion in 2025 and is expected to reach around USD 15.8 Billion by 2034, at a CAGR of 5.8% between 2025 and 2034. The key market players listed in the report with their sales, revenues and strategies are ICON plc, Parexel International Corporation, IQVIA, Covance, PRA Health Sciences, LEO Innovation Lab, Medidata, Oracle, CRF Health, Clinical Ink, Medable Inc., Signant Health, Halo Health Systems, Croprime and others.
Austin, TX, USA, Oct. 02, 2025 (GLOBE NEWSWIRE) -- Custom Market Insights has published a new research report titled “Virtual Clinical Trials Market Size, Trends and Insights By Study Design (Interventional, Observational, Expanded Access), By Indication (CNS, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Oncology, Genitourinary, Ophthalmology, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034” in its research database.
“According to the latest research study, the demand of the global Virtual Clinical Trials Market size & share was valued at approximately USD 8.8 Billion in 2024 and is expected to reach USD 8.9 Billion in 2025 and is expected to reach a value of around USD 15.8 Billion by 2034, at a compound annual growth rate (CAGR) of about 5.8% during the forecast period 2025 to 2034.”
Click Here to Access a Free Sample Report of the Global Virtual Clinical Trials Market @ https://www.custommarketinsights.com/request-for-free-sample/?reportid=74269
Overview
The Virtual Clinical Trials (VCTs) Market, according to industry experts at CMI, is registering consistent growth, owing to the growing use in the pharmaceuticals, biotechnology, and healthcare research industries. The top competitors, such as Medidata Solutions, Signant Health, and Oracle Health Sciences are improving automation, AI-powered analytics, and secure cloud applications. North America stresses regulatory compliance and modernization of clinical research infrastructure whereas Europe includes energy efficient and sustainable technology integration.
The fastest-growing region is the Asia-Pacific, which is driven by the development of healthcare, the increasing level of R&D investments, and the support of the government. The strategic partnerships between the technology providers, sponsors and research organizations are increasing the innovation, efficiency in operations and competitiveness in the market of VCTs worldwide.
Key Trends & Drivers
- Growing Stronger Demands to Decentralized Trials: As more patients choose to have a remote trial, decentralized trials lower the burden on travel, elevate retention, and provide greater access. Home-based monitoring and telemedicine are being embraced by hospitals, CROs, and pharmaceutical companies to access broader populations of patients, leading to quicker recruitment and a lower dropout rate and increasing the efficiency of the trials.
- AI and IoT Integration: Artificial intelligence and the Internet of Things enable monitoring of patients in real time, predictive analytics, and remote data capturing. These technologies increase accuracy, patient engagement, and optimization of trial protocols and adaptive trial designs. Use of smart sensors, wearable devices, and remote data streams via cloud platforms greatly reduces the need for human intervention in data collection and analysis.
Request a Customized Copy of the Virtual Clinical Trials Market Report @ https://www.custommarketinsights.com/request-for-customization/?reportid=74269
- Digitalization supports the initiative of Telemedicine: Digital experiments through telemedicine are promoted by governments and regulators alike via enabling regulations, tax breaks, and fast tracking approval. Virtual policies on remote patient monitoring, remote patient consent capturing, and autonomous e-screening enable the implementation of digital telemedicine. Defined regulations stimulate the adoption of VCTs at a larger scale by reducing compliance burdens and associated costs and building confidence among stakeholders in VCT innovation.
Report Scope
| Feature of the Report | Details |
| Market Size in 2025 | USD 8.9 Billion |
| Projected Market Size in 2034 | USD 15.8 Billion |
| Market Size in 2024 | USD 8.8 Billion |
| CAGR Growth Rate | 5.8% CAGR |
| Base Year | 2024 |
| Forecast Period | 2025-2034 |
| Key Segment | By Study Design, Indication, Phase and Region |
| Report Coverage | Revenue Estimation and Forecast, Company Profile, Competitive Landscape, Growth Factors and Recent Trends |
| Regional Scope | North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America |
| Buying Options | Request tailored purchasing options to fulfil your requirements for research. |
(A free sample of the Virtual Clinical Trials report is available upon request; please contact us for more information.)
Our Free Sample Report Consists of the following:
- The updated report for 2025 includes an introduction, an overview, and an in-depth industry analysis.
- Provide detailed chapter-by-chapter guidance on the Request.
- Updated Regional Analysis with a Graphical Representation of Size, Share, and Trends for the Year 2025
- Includes Tables and figures have been updated.
- The most recent version of the report includes the Top Market Players, their Business Strategies, Sales Volume, and Revenue Analysis
- Custom Market Insights (CMI) research methodology
(Please note that the sample of the Virtual Clinical Trials report has been modified to include the COVID-19 impact study prior to delivery.)
Request a Customized Copy of the Virtual Clinical Trials Market Report @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
SWOT Analysis
- Strengths: AI-driven analytics, IoT-based monitoring, and cloud platforms make the VCTs market efficient in terms of simplifying clinical research. Such technologies save on the expense of the trials, enhance patient recruitment and retention, and increase the accuracy of the data. The ability to meet regulatory compliance and real-time monitoring makes VCTs one of the most dependable and scalable solutions for pharmaceutical and biotech companies around the globe.
- Weaknesses: It is characterized by difficulty in incorporating the system into the wider clinical system because it requires high implementation costs, reliance on imported software/hardware, and dependencies. Smaller CROs and research centers might face limited resources, training and technological development which may slow down their deployment and decrease the efficiency benefits of the virtual trial solutions.
- Opportunities: There are opportunities for VCT growth owing to increasing demand for decentralized trials, integration of telemedicine and adoption of digital health. The new markets in Asia-Pacific and LAMEA, with the assistance of the government and the development of digital infrastructure, can be given the chance to be adopted quickly, have better access to trials, and provide cost-saving operations.
- Threats: Data privacy issues, cybersecurity threats, and regional regulatory differences are a challenge to VCT implementation. Uncertain reimbursement policies, patient reluctance toward digital environments, and rapid technological shifts can all impact the market by necessitating constant adherence to regulations, strong cybersecurity measures, and flexible platform policies.
Request a Customized Copy of the Virtual Clinical Trials Market Report @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
Key questions answered in this report:
- What is the size of the Virtual Clinical Trials market, and what is its expected growth rate?
- What are the primary driving factors that push the Virtual Clinical Trials market forward?
- What are the Virtual Clinical Trials Industry's top companies?
- What are the different categories that the Virtual Clinical Trials Market caters to?
- What will be the fastest-growing segment or region?
- In the value chain, what role do essential players play?
- What is the procedure for getting a free copy of the Virtual Clinical Trials market sample report and company profiles?
Key Offerings:
- Market Share, Size & Forecast by Revenue | 2025−2034
- Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Leading Trends
- Market Segmentation – A detailed analysis by Types of Services, by End-User Services, and by regions
- Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Buy this Premium Virtual Clinical Trials Research Report | Fast Delivery Available - [220+ Pages] @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
Regional Perspective
The Virtual Clinical Trials Market can be divided across different regions such as North America, Europe, Asia-Pacific, and LAMEA. This is a cursory overview of each region:
North America: The region continues to be an advanced market with the first da VCT adoption. Facilities and service systems are smart and efficient, thanks to the VCT regulatory AI IoT tech transdisciplinary industry amalgamation and the focusing on patient safety triad.
- United States: The whole of North America is dominated by the region due to the number of VCT platforms in place for both interventional and observational studies. Participants are more active, data accuracy increases, and engagement shifts via AI VCT analytics, with cross-cloud VCT APIs spawning trial simulators to VCT pipelines for wide data reuse.
- Canada– The adoption of virtual clinical trials (VCT) in Canada is increasing thanks to modern hospital systems, government research initiatives, telehealth, and more. Remote patient monitoring and compliance-based systems improve the trial efficiency, costs, and access for participants in rural or other underserved locations.
Europe – The health system's regulatory policies, progress on clinical trial devolution, and architectural provision of clinical decentralization in Europe explain the high adoption of VCTs in the area. Germany, the UK, and France host regional VCTs that employ AI analytics, cloud systems, and remote patient monitoring.
- Germany – The virtual and cloud hospital network systems of Germany, together with the advanced research VCTs AI analytics modular trial systems, improve patient recruitment and trial adherence while decentralizing compliance operations for the trial study.
- United Kingdom– The UK adopts more flexible clinical trial VCT platforms with predictive analytics, allowing for more scalable clinical trials of varied designs. The adoption is correlated with cloud systems that are economically and energy efficient, as well as patient-centered remote monitoring systems that relieve operational burdens.
- France– France adopts VCTs that focus on regulatory compliance and sustainable trial management. VCTs developed by hospitals, research setup, and specialized clinics succeed in patient data monitoring, data integrity assurance, and trial efficiency enhancement.
Request a Customized Copy of the Virtual Clinical Trials Market Report @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
APAC: APAC is expanding quickly because of the investments concerning healthcare, clinical infrastructure, and government policies. Chin, India, Japan and South Korea spend large amounts of money on remote monitoring tools and other digital trial platforms.
- China: In APAC, China is the aforementioned region’s leader with its broad application of VCTs in hospitals and other healthcare institutions. IoT and AI-enabled systems streamline processes like trial oversight and improve patient adherence and recruitment. They also balance government initiatives which digitize clinical research with trial digitization.
- India: Nowadays, the decentralized India’s <value chain technologies. < evolvement is facilitated by the low technology cost, strong regulatory support combined with developed hospital infrastructures. The use of telemedicine and remote monitoring in urban and peri-urban areas improves access to patients and enhances the effectiveness of studies.
- Japan: Japan is concerned with describing the value and VCT precision. The incorporation of cloud systems, AI, computer monitoring and wearable systems in hospitals and CROs improve participant safety, compliance and trial outcomes.
LAMEA: Due to support from global partners, LAMEA is gradually incorporating VCTs in participating countries like the Saudi Arabia, South Africa, and Brazil. These countries display above the curve growth.
- Brazil: Brazil’s VCT adoption hinges on continuous support from research centers, hospitals, and other decentralized driven institutions. Digital trial platforms streamline the acceleration of patients’ monitoring throughout the trials and keeping the regulations.
- Saudi Arabia: The 2030 Vision fastened the development plans aimed at integrating remote monitoring with multifunctional VCT, cloud-based trial platforms and AI analytics at the hospital and remote areas. This orthogonal optimization enhances operational efficiency and participant safety.
- South Africa: South Africa is beginning to integrate new VCT hospital technologies in research institutions. New AI, IoT, and eco-friendly systems in decentralized studies help make trials larger, keep participants more involved, and collect data more accurately
We customize your report to align with your specific research requirements. Inquire with our sales team about customizing your report.)
Still Looking for More Information? Do you want data for inclusion in magazines, case studies, research papers, or media?
Email Directly Here with Detailed Information: support@custommarketinsights.com
Browse the full “Virtual Clinical Trials Market Size, Trends and Insights By Study Design (Interventional, Observational, Expanded Access), By Indication (CNS, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Oncology, Genitourinary, Ophthalmology, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025 – 2034” Report at https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
List of the prominent players in the Virtual Clinical Trials (VCTs) Market:
- ICON plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable Inc.
- Signant Health
- Halo Health Systems
- Croprime
- Others
Click Here to Access a Free Sample Report of the Global Virtual Clinical Trials Market @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
Spectacular Deals
- Comprehensive coverage
- Maximum number of market tables and figures
- The subscription-based option is offered.
- Best price guarantee
- Free 35% or 60 hours of customization.
- Free post-sale service assistance.
- 25% discount on your next purchase.
- Service guarantees are available.
- A personalized market brief by the author.
Browse More Related Reports:
Digital Mammography Market: Digital Mammography Market Size, Trends and Insights By Technology (2D Full Field Digital Mammography, 3D Full Field Digital Mammography (Digital Breast Tomosynthesis), Contrast Enhanced Digital Mammography), By Product Type (Digital Mammography System, Display Unit, Central Processing Units, Visualization Software), By End-user (Hospitals and Surgical Centers, Breast Care Centers, Diagnostic Centers), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Paracetamol Market: Paracetamol Market Size, Trends and Insights By Product Type (Tablet, Capsule, Liquid Suspension, Powder), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), By Application Type (Headache & Fever, Muscle Cramps, Cold & Cough), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Lenacapavir Injection Market: Lenacapavir Injection Market Size, Trends and Insights By Indication (HIV Treatment, Pre-Exposure Prophylaxis (PrEP)), By Formulation (Injectable, Oral Tablets), By Distribution Channel (Branded Medicine, Generic Medicine), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Pharmaceutical Metal Detector Market: Pharmaceutical Metal Detector Market Size, Trends and Insights By Technology (Magnetic Field Detectors, Multi-frequency Detectors, X-Ray Inspection with Metal Detectors), By Application (Quality Control, Contaminant Detection, Packaging, Compliance), By End-user (Pharmaceutical Companies, Contract Development and Manufacturing Organizations (CDMOs), Biopharmaceutical Companies), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Medical Equipment Calibration Services Market: Medical Equipment Calibration Services Market Size, Trends and Insights By Service (In-house, Third-party Services, Original Equipment Manufacturers (OEMs)), By End-use (Hospitals, Clinical Laboratories), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
CRISPR and Cas Genes Market: CRISPR and Cas Genes Market Size, Trends and Insights By Technology Type (CRISPR/Cas9, CRISPR/Cas13, CRISPR/Cas14, Others), By Delivery Method (Ex vivo, In vivo, Physical Methods, Others), By Application Area (Functional Genomics, Oncology, Infectious Diseases, Others), By Tool Type (Guide RNA, CRISPR Plasmids, Cas Nucleases, Custom CRISPR Libraries, Vectors & Cloning Kits), By Crop Type (Cereals & Grains, Fruits & Vegetables, Oilseeds & Pulses, Industrial Crops), By Disease Type (Cancer, Blood Disorders, Ophthalmic Diseases, Infectious Diseases, Others), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Y Chromosome Microdeletion Detection Kit Market: Y Chromosome Microdeletion Detection Kit Market Size, Trends and Insights By Product Type (PCR-based Kits, FISH-based Kits, Others), By Application (Hospitals, Diagnostic Laboratories, Research Institutes, Others), By End-User (Healthcare Providers, Research Laboratories, Others), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
CRISPR Gene Editing Market: CRISPR Gene Editing Market Size, Trends and Insights By Product (Kits and Reagents, Services), By Gene Editing Modality (Ex-Vivo Editing, In-Vivo Editing), By Technology (CRISPR/Cas9 Technology, CRISPR/Cas12 Technology, Prime Editing, Epigenetic Editing, Others), By Application (Therapeutic Applications, Agriculture and Livestock, Industrial Biotechnology), By End User (Biotechnology and Pharmaceutical Companies, Agricultural and Livestock Industry, Hospitals and Clinics, Others), and By Region - Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
The Virtual Clinical Trials (VCTs) Market is segmented as follows:
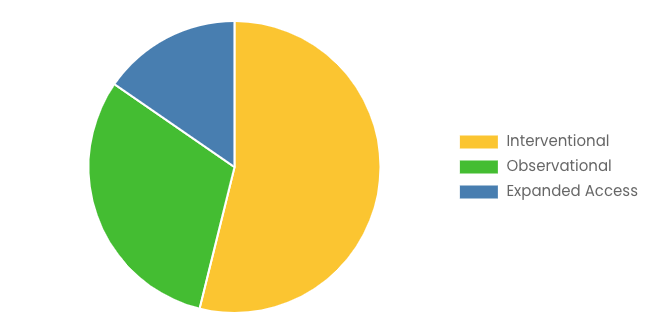
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- CNS
- Autoimmune/Inflammation
- Cardiovascular Disease
- Metabolic/Endocrinology
- Infectious Disease
- Oncology
- Genitourinary
- Ophthalmology
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Click Here to Get a Free Sample Report of the Global Virtual Clinical Trials Market @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
Regional Coverage:
North America
- U.S.
- Canada
- Mexico
- Rest of North America
Europe
- Germany
- France
- U.K.
- Russia
- Italy
- Spain
- Netherlands
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Taiwan
- Rest of Asia Pacific
The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America
This Virtual Clinical Trials Market Research/Analysis Report Contains Answers to the following Questions.
- Which Trends Are Causing These Developments?
- Who Are the Global Key Players in This Virtual Clinical Trials Market? What are the company profiles, product information, and contact details for these key players?
- What Was the Global Market Status of the Virtual Clinical Trials Market? What Was the Capacity, Production Value, Cost, and PROFIT of the Virtual Clinical Trials Market?
- What Is the Current Market Status of the Virtual Clinical Trials Industry? What's the market's competition in this industry, both company-wise and country-wise? What's Market Analysis of Virtual Clinical Trials Market by Considering Applications and Types?
- What Are Projections of the Global Virtual Clinical TWhat is the market analysis of the Virtual Clinical Trials industry, considering its applications and types? Cost and Profit? What Will Be Market Share, Supply, and Consumption? What about imports and exports?
- What is a Virtual Clinical Trials estimatein analysis of upstream raw materials and downstream industries?
- What is the economic impact on the Virtual an analysis of the market chain for Virtual Clinical Trials, including Environment Analysis Results? What Are Global Macroeconomic Environment Development Trends?
- What Are the Market Dynamics of the Virtual Clinical Trials Market? What Are Challenges and Opportunities?
- What Should Be Entry Strategies, Countermeasures to Economic Impact, and Marketing Channels for Virtual Clinical Trials Industry?
Click Here to Access a Free Sample Report of the Global Virtual Clinical Trials Market @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
Reasons to Purchase Virtual Clinical Trials Market Report
- Virtual Clinical Trials Market Report provides qualitative and quantitative analysis of the market based on segmentation involving economic and non-economic factors.
- Virtual Clinical Trials The Market report outlines market value (USD) data for each segment and sub-segment.
- This report indicates the region and segment expected to witness the fastest growth and dominate the market.
- Virtual Clinical Trials Market Analysis by geography highlights the consumption of the product/service in the region and indicates the factors affecting the market within each region.
- The competitive landscape incorporates the market ranking of the major players, along with new service/product launches, partnerships, business expansions, and acquisitions in the past five years of companies profiled.
- Extensive company profiles comprise a company overview, company insights, product benchmarking, and SWOT analysis for the major market players.
- Recent developments, including growth opportunities and drivers, as well as challenges and restraints in both emerging and developed regions, shape the industry's current and future market outlook.
- Virtual Clinical Trials Market: Includes in-depth market analysis from various perspectives through Porter's five forces analysis and offers an overview of the market through the value chain.
Reasons for the Research Report
- The study provides a thorough overview of the global Virtual Clinical Trials market. Compare your performance to that of the market as a whole.
- Aim to maintain competitiveness while innovations from established leaders drive market growth.
Buy this Premium Virtual Clinical Trials Research Report | Fast Delivery Available - [220+ Pages] @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
What does the report include?
- Drivers, restrictions, and opportunities are among the qualitative elements covered in the worldwide Virtual Clinical Trials market analysis.
- The report covers the competitive environment of current and potential participants in the Virtual Clinical Trials market, along with their strategic product development ambitions.
- This study conducts a qualitative and quantitative analysis of the Virtual Clinical Trials market based on the component, application, and industry vertical. Additionally, the report provides comparable data for the key regions.
- The report provides actual market sizes and forecasts for each segment mentioned above.
Who should buy this report?
- Participants and stakeholders worldwide Virtual Clinical Trials market should find this report useful. The research will be useful to all market participants in the Virtual Clinical Trials industry.
- Managers in the Virtual Clinical Trials sector are interested in publishing up-to-date and projected data about the worldwide Virtual Clinical Trials market.
- Governmental agencies, regulatory bodies, decision-makers, and organizations want to invest in Virtual Clinical Trials products' market trends.
- Analysts, researchers, educators, strategy managers, and government organizations seek market insights to develop plans.
Request a Customized Copy of the Virtual Clinical Trials Market Report @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/
About Custom Market Insights:
Custom Market Insights is a market research and advisory company delivering business insights and market research reports to large, small, and medium-scale enterprises. We assist clients with strategies and business policies and regularly work towards achieving sustainable growth in their respective domains.
CMI is a one-stop solution for data collection and investment advice. Our company's expert analysis digs out essential factors that help us understand the significance and impact of market dynamics. The professional experts advise clients on aspects such as strategies for future estimation, forecasting, opportunities to grow, and consumer surveys.
Follow Us: LinkedIn | Twitter | Facebook | YouTube
Contact Us:
Frank Gittens
CMI Consulting LLC
1333, 701 Tillery Street Unit 12,
Austin, TX, Travis, US, 78702
USA: +1 737-734-2707
APAC: +91 20 46022736
Email: support@custommarketinsights.com
Web: https://www.custommarketinsights.com/
Blog: https://businessresearchindustry.com
Blog: https://www.briinsights.com/
Blog: https://cmimarketresearch.com/
Buy this Premium Virtual Clinical Trials Research Report | Fast Delivery Available - [220+ Pages] @ https://www.custommarketinsights.com/report/virtual-clinical-trials-market/

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.